Alkalinity: Essential for Coral Health and Aquarium Stability
In aquarium keeping, water chemistry plays a vital role in the health and stability of your aquatic environment. One key component is alkalinity, or carbonate hardness (KH), which refers to the water’s buffering capacity—its ability to resist changes in pH. This stability is especially important in both freshwater and marine tanks, as sudden pH shifts can harm fish, plants, and corals, which thrive in consistent conditions. Ideal KH levels vary based on aquarium type: for reef aquariums, KH should range between 7 and 11 dKH, with 8-9 dKH often recommended, while freshwater tanks do best around 4-8 dKH, with species-specific needs such as lower KH for Amazonian fish (1-4 dKH) and higher KH for livebearers (4-8 dKH).
The Role of Alkalinity in Aquariums
Alkalinity acts as a “shock absorber” for pH, keeping it steady and creating a suitable environment for aquatic life. For instance, corals, which are sensitive to even slight pH fluctuations, depend on consistent alkalinity for a critical process called biomineralization or calcification. During calcification, corals use calcium and carbonate ions to build their calcium carbonate skeletons. KH represents the concentration of bicarbonate ions in the water, which can be converted into carbonate ions as needed in the calcification process. This ensures that corals have a steady supply for skeleton formation, but if pH drops too low, the availability of these ions decreases, which can slow or even reverse growth.
In freshwater tanks, KH also stabilizes pH, supporting plant growth and keeping fish stress-free. However, marine aquariums require more precise KH control because corals and other invertebrates are particularly sensitive to changes.
Conventional Methods for Measuring KH
Many aquarists measure KH using manual test kits or digital titration devices. Manual test kits, which involve adding reagents to a water sample and observing a color change, are affordable and widely available. However, they can be prone to user error, and repeated testing can be time-consuming. Digital titration devices offer improved accuracy but still require manual sampling and tend to be costlier. Additionally, both methods lack reliably consistent, on-scheduled monitoring, which means that significant KH shifts could go undetected between tests, impacting the health of sensitive organisms like corals.
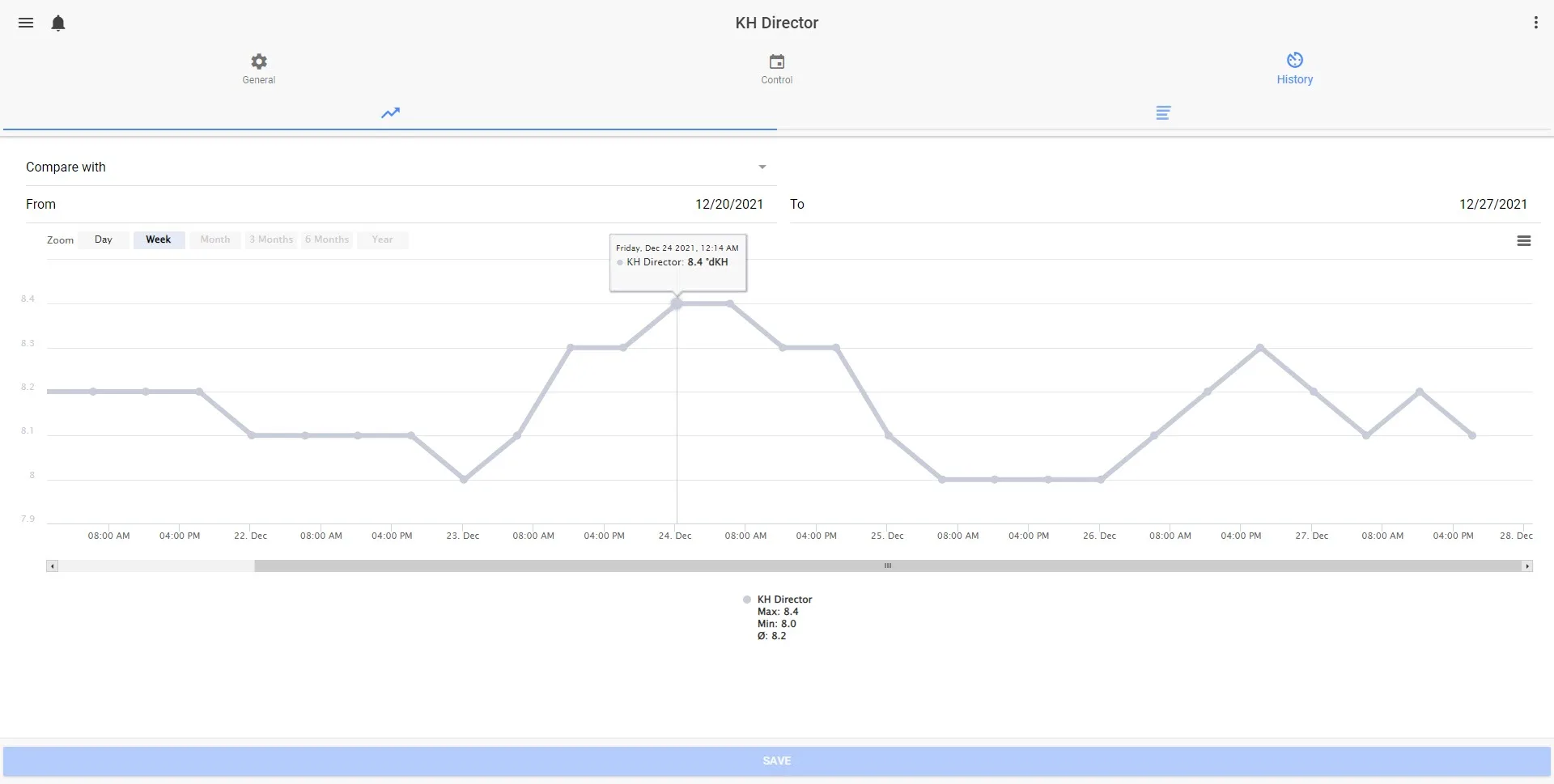
The Benefits of Automated KH Monitoring with KH Director
For aquarists seeking more control and ease of use, automated tools like the KH Director provide a highly effective alternative. Designed for flexibility, the KH Director from GHL can be used as a standalone unit or in conjunction with the ProfiLux 4 controller. When connected to a ProfiLux 4, the KH Director can synchronize with monitoring other water parameters and dosing processes, enhancing overall aquarium stability by automating adjustments across multiple water parameters.
The KH Director excels in precision and reliability, offering a repeatability of 0.1 dKH – level of accuracy that helps maintain the exact alkalinity levels necessary for healthy coral calcification. This high level of precision is crucial, as even slight changes can affect coral resilience and growth.
Additionally, the KH Director connects with the GHL Connect app and PC software, which provide visual charts for tracking alkalinity over time. This functionality enables aquarists to monitor KH trends and make informed adjustments when needed. Alerts can also be set up to notify users of any deviations from the target range, giving them peace of mind and allowing them to address potential issues proactively.
In summary, the KH Director’s automated management of alkalinity ensures a balanced, low-maintenance reef or freshwater aquarium, where corals or other underwater life can thrive. This tool provides professional aquarists with the accuracy, ease, and reliability needed for optimal aquarium health, making it a valuable addition to any aquarium setup.
And what is now the difference between KH and alkalinity?
KH (carbonate hardness) and alkalinity are closely related concepts in aquarium water chemistry, but they are not exactly the same thing. Here’s an explanation of their relationship and differences:
Alkalinity
Alkalinity is the water’s capacity to neutralize acids. It’s a measure of the concentration of negative ions in the water that can bind to hydrogen ions (protons), thus resisting changes in pH. These ions include carbonates (CO3²⁻), bicarbonates (HCO3⁻), borates (BO3³⁻), and phosphates (PO4³⁻).
KH (Carbonate Hardness)
KH, despite its name, is not actually a measure of hardness but rather a measure of alkalinity. In aquarium contexts, KH is often used interchangeably with alkalinity because:
- In most natural waters with pH below 8.4, over 90% of the alkalinity comes from bicarbonates.
- Aquarium test kits labeled as “KH” are actually measuring total alkalinity.
Key Differences and Similarities
- Composition: While alkalinity includes all acid-neutralizing compounds, KH specifically refers to carbonates and bicarbonates.
- Measurement: Both KH and alkalinity are typically expressed in the same units (dKH, meq/L, or ppm CaCO3).
- Practical Use: In most aquarium situations, KH and alkalinity values will be very close or identical.
- Exceptions: In some cases, particularly with treated water or certain additives, alkalinity might be higher than what a KH test indicates due to the presence of other buffering compounds.